String-theory has exactly the same problems, but the other way round. There are only all the possibilities, but no robustness at all. Only mind without body? Can mind be part of the body? Can the problem be solved, and interactions (measurements) be done?

What happen when we do interactions/measurements of our surroundings? If we want to scale down the question we must look at the cell. How do the cell experience its surroundings? There is probably very little difference to the cell if the measurement comes from the own body or from environment. How do the cell differ of meaningful and meaningless (noise) signals? Where can we talk of stress? How do the cell handle too much stress?
If we leave the cell psychology ahead for a while, and look at the surroundings, the perceptions, and the magnetic impact. The cell psychology is as instance the qualia problem, but we have no tools yet to discuss that. We must be reductionistic in this situation.
Membranes in biology.
Membranes are bipolar lipids, with a low dielectric constant (due to fat) and a content of other molecules that can be blocked or drawn from the membrane when they are not wanted or needed. They are phagocytosed mostly. The lipid membrane is floating, loose, and an energy reservoir for phosphorylation, and at the same time a communication tool for the neighbourhood (exocrinal hormonal cell signalling, paracrine and autocrine hormonal signals), sense organs for the cell, etc.
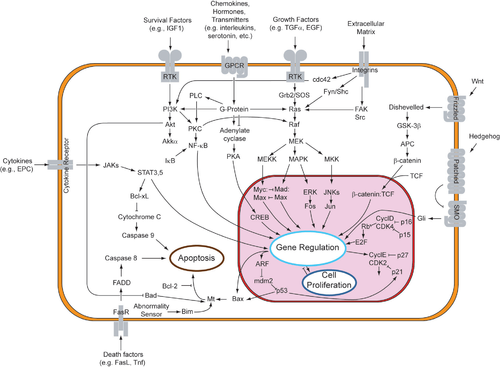 Overview of signal transduction pathways. Wikipedia. Look only at the mess.
Overview of signal transduction pathways. Wikipedia. Look only at the mess.Some signals can pass through the membrane without 'passcode', as important ones like oestrogen, insuline, thyroxine, other need a multiply of 'tests' before they are allowed to pass. A second messenger such as Ca++ or cAMP is needed. Then there are other membranes, as the nuclear envelope, that also control it's 'passports'. In principle everything that happens is part of the control output of the cell. There can be no uncontrolled things 'that just happen'.
The 'passport' can also be activated in other ways. For example, the neurotransmitter GABA can activate a cell surface receptor that is part of an ion channel. However, for many cell surface receptors, ligand-receptor interactions are not directly linked to the cell's response. The activated receptor must first interact with other proteins inside the cell, in a signal transduction mechanism or pathway.
An external signal gives conformal changes in a protein-chain interaction, the mitotic cell cycle is involved, receptors that are kinases start phosphorylation of themselves or others, and induce growth, etc. The adaptor proteines do the choise, the jump. The phosphorylated receptor binds to an adaptor protein , which couples the signal to further downstream signaling processes, for instance attach phosphate to target proteins, and alter cell cycle progression, or output. Complex multi-component signal transduction pathways provide opportunities for feedback, signal amplification, and interactions inside one cell between multiple signals and signaling pathways. What decides the choise done by the cell? Has the cell a free will? As we see many of these steps are also quantum biological. Has the quantum biology, the holographic body, any meaning for the cell?
This is a very simplified picture, where I try to point at essential features only. You can see at a sample of communication ways below. I cannot go into depth into the extremengly interesting signalling this time. You only need to know how complicated it is.
- Molecular Cellular Cognition
- Crosstalk (biology)
- MAPK signaling pathway
- Hedgehog signaling pathway
- TGF beta signaling pathway
- JAK-STAT signaling pathway
- cAMP dependent pathway
- Protein dynamics
- Signal transduction
- Systems biology
- Semiotics
- Lipid signaling
- Redox signaling
A. The cell membrane.
The cell membrane is more a loci only. A place where signals arrive. Adey, one of the pioneers and big names in this field say 1988 in 'Cell Membranes: The Electromagnetic Environment and Cancer Promotion',
...the sequence and energetics of events that couple humoral stimuli from surface receptor sites to the cell interior has identified cell membranes as a primary site of interaction with these low frequency fields. Field modulation of cell surface chemical events indicates a major amplification of initial weak triggers associated with binding of hormones, antibodies and neurotransmitters to their specific binding sites. Calcium ions play a key role in this stimulus amplification, probably through highly cooperative alterations in binding to surface glycoproteins, with spreading waves of altered calcium binding across the membrane surface. Protein particles spanning the cell membrane form pathways for signaling and energy transfer. Fields millions of times weaker than the membrane potential gradient of 10^5 V/cm modulate cell responses to surface stimulating molecules. The evidence supports nonlinear, nonequilibrium processes at critical steps in transmembrane signal coupling. Powerful cancer-promoting phorbol esters act at cell membranes to stimulate ornithine decarboxylase which is essential for cell growth and DNA synthesis. This response is enhanced by weak microwave fields, also acting at cell membranes.
Adey says that cell membranes, in coupling humoral stimuli (hormones, neurotransmitters and antibodies) from surface receptor sites to the cell interior, functions as a primary site of interaction with weak oscillating EM fields in the pericellular fluid. This would mean that the primary signal comes not from the cell alone, but from the cell environment. And the signal is forcefully amplified in the passage through the membrane. This we know is true, but not in every case. The important GCPR-receptors stand out here, making up for about half of all receptors. They are most often the targets for the drugs. EM fields in fluid surrounding cells modulate inward and outward signal streams through cell membranes. Is this the real impact? He writes:
Careful evaluation of these field actions has revealed subtle effects that betoken mechanisms of interaction based on long range interactions and nonequilibrium processes. Temperature increments are not the primary substrates of the observed biological sensitivities.
This was tested for lymphoid cells (leukemia) in 1995. Electromagnetic waves (1G, 60 Hz -this is quite high stimulus level) stimulates the protein tyrosine kinases, so that it results in tyrosine phosphorylation of multiple electrophoretically distinct substrates, and leads to downstream activation of protein kinase C (PKC). A wave of 'destruction' into the cell. But this destruction is selective, some kinases are stimulated, and a delicate growth regulatory balance might be altered.
In fact there are many studies revelaing a link between cancer and EM-fields. Humanmade fields are substantially above the naturally occurring ambient electric and magnetic fields of ~10^-4 Vm^-1 and ~10^-13 T, respectively. Several epidemiological studies have concluded that ELF-EMFs may be linked to an increased risk of cancer, particularly childhood leukemia. How might EMFs induce cancer?
Magnetic fields can also change the opioid levels, by modulating the gene expression.
Ventura et al. writes:
Magnetic fields have been shown to affect cell proliferation and growth factor expression in cultured cells. Although the activation of endorphin systems is a recurring motif among the biological events elicited by magnetic fields, compelling evidence indicating that magnetic fields may modulate opioid gene expression is still lacking. We therefore investigated whether extremely low frequency (ELF) pulsed magnetic fields (PMF) may affect opioid peptide gene expression and the signaling pathways controlling opioid peptide gene transcription in the adult ventricular myocyte, a cell type behaving both as a target and as a source for opioid peptides.Vetura, cont.
Conclusions: The present findings demonstrate that an opioid gene is activated by myocyte exposure to PMF and that the cell nucleus and nuclear embedded PKC are a crucial target for the PMF action. Due to the wide ranging importance of opioid peptides in myocardial cell homeostasis, the current data may suggest consideration for potential biological effects of PMF in the cardiovascular system.
Magnetic fields may elicit multiple effects in biological systems, including behavioural changes in intact organisms. Effects of MF on opioid-related events may have important implications in cellular homeostasis. Among the regulatory systems that appear to be targeted are endogenous opioid peptides. MF can produce analgesic effects through an opioid receptor-mediated mechanism and are able to affect the spontaneous electrical brain activity by interfering with the action of both exogenous and endogenous opioids. In mice, MF have been found to enhance the duration of pharmacologically-induced anaesthesia by releasing endogenous opioids and/or enhancing the activity of opioid signaling pathways. The capability of MF of controlling the central cholinergic system also appears to depend on the activation of an opioidergic pathway. Opioid receptor antagonism also attenuated MF-induced antiparkinsonian effects in man. Opioid peptides may act as growth modulators and may control both cell differentiation and architecture in a wide variety of tissues. The myocardial cell responds to opioid receptor stimulation with deep changes in cytosolic Ca2+/pH homeostasis and contractility. Dynorphin B released Ca2+ from an intracellular store acted in an autocrine fashion to stimulate the transcription of its coding gene (for dynorphin B), involving an impairment of cell growth and differentiation. A delicate growth regulatory balance may be altered following nuclear PKC activation by PMF. PMF-induced prodynorphin gene transcription resulted in the increase of both intracellular and secreted dynorphin B. Dynorphin B is known to bind selectively opioid receptors and the stimulation of these receptors in cardiac myocytes has been shown to promote phosphoinositide turnover, depletion of Ca2+ in the sarcoplasmic reticulum and leading to a marked decrease in the amplitude of the cytosolic Ca2+ transient oscillations and in that of the associated contraction of the heart. Ventura et al ask: Why would hearth cells have a system capable of reacting to PMF? In isolated nuclei an opioid gene can be independently and fully activated by PMF, as in the intact cell. The property of conveying nuclear signaling to the modulation of gene transcription may disclose new perspectives in the molecular dissection of the biological effects.
Magnetic fields may
- alter human cardiac rhythm (Ventura et al.)
- enhance the occurrence of arrhythmia-related heart problems (Ventura et al.)
- induce stress responses that protect the embryonic myocardium from anoxia damage (chick)
- influence the spontaneous electrical brain activity (rat) (Vorobyov et al.1998.) consistent with the findings of other groups demonstrating that weak magnetic fields may drastically modify the effects of both exogenous and endogenous opioids on different basic functions in vertebrates and invertebrates.
Lithium- and dopamine effects.
Modification of a brain opioid system may contribute to the clinical response to lithium. Li increase the phosphorylation, but not in the presence of Ca2+ or Ca2+ and calmodulin. Chronic lithium treatment affects some signal transduction mechanisms such as cAMP, cGMP, inositol 1,4,5 P3, Gi protein, protein kinase C and can also modify gene expression in rat brain. Li affects the adrenoceptors and their half lives (=turnover rate). Administration of Li is associated with a reduction in retinal light sensitivity, but chronic lithium use is not associated with differences in retinal light sensitivity, and no retinal toxicity is feared. Li diminish neostriatal dopaminergic activity, but the underlying mechanisms do not appear to involve modifications in either the D1 or the D2 receptor primary ligand recognition sites. The hypothesis of an increased dopamine synthesis is not supported and Li modified the affinity of DA transporters for the radioligand, possibly a consequence of conformational changes induced by the disruption of the nerve terminal membrane environment. Lithium blocks isolation-induced hypersensitivity, especially of the β-adrenergic system. Isolation reduce motor activity, seen in rats. Lithium has an inhibitory effect on neuroleptic receptors ([3H]spiroperidol binding sites) in the limbic-forebrain and on serotonin receptors ([3H]serotonin binding sites) in the hippocampus. Serotonin directs the attention amongst others. The effect of lithium ion on the electrically stimulated 5-[3H]hydroxytryptamine (5-HT) release from the rat hippocampal decreased when exposed to 5-HT, but Li did not affect release alone but inhibited together with serotonin. Li may inhibit the regulation of 5-HT release via presynaptic 5-HT autoreceptors in rat hippocampus.
The response on penile erection induced by apomorphine, a mixed Dl/D2 dopamine receptor agonist, (0.05-0.5 mg/kg), was decreased in animals pretreated with chronic lithium, and inhibitory effect of sulpiride increased too. No bliss with Li.
Serotonergic (5-HT) dysfunction has been hypothesized in mania, but the results are inconsistent. The platelet 5-HT2 receptor is neither a state marker nor a trait marker in mania, and maybe the serotonin hypothesis is wrong. There are though a clear up- or down-regulation of platelet serotonin receptor responsiveness in bipolar and unipolar depression.
The effect on second messengers is interesting too. Lithium reduced the inhibitory ability of carbachol, and reduced the degree of stimulation of formation of inositol phosphate, induced by noradrenaline. Chronic effects of administration of lithium may be related to actions at the G protein level and that different modes of coupling of receptors to G proteins may be responsible for the variety of effects observed.
A selective D1 dopamine receptor antagonist, blocked an increase in cAMP formation of all of the dopamine agonists investigated. Are there a relationship between the D1 receptor-stimulated increase in cAMP formation and the induction of dyskinesia in Parkinsonian humans? Robust catalepsy follow from D1 receptor blockade (rat), while dopamine agonists (as apomorphine) effects on bradycardia (induced by stim vagus nerve) decreased significantly the vagal nerve -induced (but blocked by sulpiride) but not the acetylcholine-induced bradycardia, and suggest the presence of presynaptic and/or ganglionic dopamine DA2 receptors in the parasympathetic innervation of the rat heart, stimulation of which inhibits the release of acetylcholine. As a parenthesis I must say Li is mostly used to prevent mania. Stork & Renshaw, 2005, propose a hypothesis of mitochondrial dysfunction in bipolar disorder that involves impaired oxidative phosphorylation, a resultant shift toward glycolytic energy production, a decrease in total energy production and/or substrate availability, and altered phospholipid metabolism.
Dopaminergic and opioidergic systems interact in the striatum in the brain to modulate locomotor and motivated behaviors. Dopamine modulate opioid receptor-mediated signal transduction. Repeated activation of D1 receptors attenuates the functional coupling of delta opioid receptors with adenylyl cyclase due to decreased coupling between delta receptors and G proteins.
Li acts through cyclotrone resonanse frequencies, as Ca, and Fe do? It's secrets cannot be revealed on cellular level?
Age and cAMP-production.
Blood vessels from aged animals and humans have impaired relaxation and cAMP production to β-adrenergic stimulation, but direct activators of adenylyl cyclase are not affected. Would the effects on cAMP production occur in membrane? Aortic media membrane was studied in rats. Basal AC activity increased significantly with age, but no age-related decrease in responsiveness for G protein activators, or receptor agonists β-adrenergic and PGE-1 (prostaglandin). The membrane system to assess age-related changes in β-adrenergic responsiveness seem not be the case. Cocaine reduce cAMP production, as age do. A functional change in a critical signal transduction pathway and effects the development of the brain.
Overall membrane charachters.
So, it seem it is not the membrane that is magnetically active, but the receptors, and they may be activated through an magnetic attraction of the second messengers cAMP, Ca++, GTP etc. These second messengers then amplify the signal.
But the membrane give very clear response in magnetic induction fields, seen in fMRI. The cell membrane and especially its receptors, acts as a capture for magnetic waves, just as the genes are captures, seen in the promoter genes. The magnetic field (weak permanent homogenous hirizontal magnetic field (PMF) 400 A/m) affects the lipid constitution too. In radish seedlings Novitskaya et al. found that PMF increased the ratio of phospholipids to sterols by 30–100%, and suppressed the formation of polar lipids in light (by 18%), whereas in darkness, it stimulated it approximately by 80%, very temperature dependant. PMF exerted the strongest effect on the content of erucic acid. PMF behaved as a correction factor affecting lipid metabolism on the background of light and temperature action. Membrane composition also varies between vertebrates and the degree of polyunsaturation of membrane phospholipids is correlated with cellular metabolic activity, so that more phospholipids give a faster metabolism. Membranes can act as pacemakers for overall metabolic activity. Such membrane polyunsaturation increases the molecular activity of many membrane-bound proteins and consequently some specific membrane leak–pump cycles and cellular metabolic activity. A greater transfer of energy during intermolecular collisions of membrane proteins with the unsaturated two carbon units (C=C) of polyunsaturates compared to the single carbon units of saturated acyl chains, as well as the more even distribution of such units throughout the depth of the bilayer when membranes contain polyunsaturated acyl chains compared to monounsaturated ones. The proposed pacemaker role of differences in membrane bilayer composition have importance to the brain (and sensory cells), evolution of mammalian endothermic metabolism, etc.
When a cell is exposed to a time-varying magnetic field, this leads to an induced voltage on the cytoplasmic membrane, as well as on the membranes of the internal organelles, such as mitochondria. These potential changes in the organelles could have a significant impact on their functionality. The amount of polarization in the organelle was less than its counterpart in the cytoplasmic membrane. This was largely due to the presence of the cell membrane, which "shielded" the internal organelle from excessive polarization by the field. Organelle polarization was largely dependent on the frequency of the magnetic field.

Regional polarization of the cytoplasmic membrane and the organelle membrane by the time-varying magnetic field. The plot demonstrated an instant polarization pattern on both membranes. The color map represented the amount of polarization (in mV) calculated with the standard values listed in table 1. A. Field frequency was 10 KHz. B. Field frequency was 100 KHz. Ye et al. 2010.
The effect is also seen in a pattern generation of the molecules in the cell membrane. Distinct 'fields' are clearly seen. This is partly a result of chemical attractions, but also electric and magnetic. The danish solitonic nerve pulse model clearly show such patterns.

Electric field vector plot and potential distribution near the plasma membrane with mobile surface charges in an alternating electric field. The uniform electric field in the cell is greater at (a) 106 Hz than at (b) 102 Hz. The excitation field is 1.0 V/cm. Vajrala et al. 2008. Observe that this is an electric field.
Elastic fibres have important cell adhesion functions. Electron microscopy and biochemical studies have highlighted strong interaction with their subendothelial elastic fibre-containing matrix, and with juxtaposed elastic fibre lamellae at cell surface dense plaques. These interactions are mediated mainly through heterodimeric transmembrane receptors.
Many diseases depends on the microtubuli - attatchment to the cell membrane. Myotrophies as Duchennes and Beckers dystrofies are one result. Without a proper cytosceleton the cell cannot work.
B. How might EMFs induce cancer?
Lacy-Hulberta et al. writes:
Free radicals are generated as intermediates in metabolism and may attack lipids, proteins, and DNA. Thus, any elevation in free radical production could increase the rate of chemical damage to DNA as occurs, for example, as a consequence of sustained activation of the immune system in response to chronic infection. Magnetic fields of more than 1 mT can have measurable effects on the kinetics and yield of chemical reactions that use geminate radical pairs through their effect on the spin precession rates of unpaired electrons and consequent effects on the lifetime of radicals. The magnetic field can increase or decrease precession rates between singlet and triplet spin-correlated states. Hence, a geminate radical pair born in the singlet spin state may rapidly recombine; after precession to the triplet spin state, recombination is prohibited by the Pauli exclusion principle, resulting in a longer radical lifetime. The consequence of this may be, for example, increased enzyme product or release of radicals from the enzyme.

Electromagnetic field effects on free radical processes. A) A reaction between two species can generate a pair of radicals in the triplet state with parallel electron spin. If one of the electrons converts to a different state, changing its spin, the radical pair can react to form product. B) This change involves transfer of the electron between the three triplet states: T0, T-1, and T+1. These states are normally degenerate, but in a magnetic field the energies separate. When this separation is less than the hyperfine reaction for the system, the radicals created in the triplet states can be transformed into singlets and react. When the separation is greater than the hyperfine reaction, radicals created in T-1 and T+1 triplet states cannot interconvert and hence reaction cannot take place. However, an alternating magnetic field of frequency {upsilon} can excite electron transitions between levels, allowing transition to the singlet states even in high magnetic fields.
Alternating magnetic fields superimposed on static magnetic fields can further affect reactions by providing quanta of energy equal to the gap between singlet and triplet states, allowing transition of radicals and hence increasing reaction probability. The effect requires both static magnetic fields and fields fluctuating at a resonance frequency. These examples (ROS, neutrophiles) represent clear, reproducible effects of magnetic fields on biochemical systems with a firm theoretical basis. Effects are reported from 0.1 mT fields at 60 Hz. Nitric oxide production is also interesting, as other immune reactions.
Lacy-Hulberta:
A free radical basis for magnetic field effects would have some important implications for investigations and epidemiological studies. The processes affected occur very rapidly, and so at the level of simple effects are independent of frequency; in many cases, the geomagnetic field exposure would far outweigh the alternating field. However, as described above, more complex effects can occur in vitro with specific combinations of static and alternating magnetic fields, and these combinations vary with the free radical species involved.
This also correlates with the radical-pair theory of Ritz mentioned in earlier posting. Also with the laser-effects found by Tiina Karu. Seedling growth magnetically sensitive as a result of photoinduced radical-pair reactions in cryptochrome photoreceptors—tested by measuring several cryptochrome-dependent responses, all of which proved to be enhanced in a magnetic field of intensity 500 μT, show a way forward.
C. The Extracellular Matrix (ECM).
Disturbances and dysfunctions are most evident effects of longterm illness, and medicines seldom can change this. Is the reason somewhere else? In the synchronisation/control-levels, but not at cell-level? To believe something else only means misuse of medicine? We need to look at the networks, to step up a level in the molecular hierarchies. Maybe the reason to the induced control-signals are found there?
We have seen that many of the signals indeed arrive from outside the cell, even gene regulation signals. When we look at the SRP-molecule in the promoter-gene we find all those loops that will change the magnetic field very strongly, make superpositions as Lacy-Hulberta et al. suggests.
In fact, Nature herself use networks, as seen in nerves, blood circulation, gap junction systems, nanotubes, hormonal systems, meridians etc. Maybe there are an oxygen network too, as Mae Wan Ho said? Oxygen makes more energy available and at greater efficiency; at the same time, it increases the complexity of metabolic networks. A network that take part very much in the relaxation in form of negentropic bindings. A fast relaxation is important for the functions in the organisms. They are as important as the very minute signal is. In fact,it is exactly the relaxation that make up the robustness and criticality in matter. It is the probabilities that changes in different magnetic fields, The relaxation leads to polarization. Adhesion and adsorption makes it go faster and time is very important too.
Paluch et al. writes 2006:
The shape of animal cells is, to a large extent, determined by the cortical actin network that underlies the cell membrane. Because of the presence of myosin motors, the actin cortex is under tension, and local relaxation of this tension can result in cortical flows that lead to deformation and polarization of the cell. Cortex relaxation is often regulated by polarizing signals, but the cortex can also rupture and relax spontaneously. A similar tension-induced polarization is observed in actin gels growing around beads, and we propose that a common mechanism governs actin gel rupture in both systems.
We shall look at yet a network, the extracellular matrix, that do supramolecular organizations, and is non-local and very fast. But that will be in a new posting, 'Stress and relax. The Extracellular Matrix. Brain modelling VIII b', coming soon.
I will finish with some words from Matti Pitkänen.
In this framework the energy feed to the system means that the (quantum) superposition changes in such a manner that the average energy of the positive energy state increases. This excites new degrees of freedom and makes the system more complex. The dissipation caused by quantum jumps reducing entanglement entropy tends to reduce the average energy and this tendency is compensated by the energy feed selecting also the most stable self-organization patterns as a flow equilibrium.
The hologrammic organization is the ultimate, most stable organization. But in a holistic model ought the gravity also be included. Maybe we will soon know what gravity really is?
References:
W.R.Adey 1988: Cell Membranes: The Electromagnetic Environment and Cancer Promotion. Neurochemical Research, Vol. 13, No. 7, 1988, pp. 671-677. http://www.springerlink.com/content/h507p8wq85141871/fulltext.pdf?page=1
W. Ross Adey 1993: Biological Effects of Electromagnetic Fields. Journal of Cellular Biochemistry 51:410-416 (1993). http://www.energycelltherapy.co.uk/pdfs/biological.pdf
Sue-Re Harris, Kevin B. Henbest, Kiminori Maeda, John R. Pannell, Christiane R. Timmel, P.J. Hore and Haruko Okamoto, 2009: Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 6 December 2009 vol. 6 no. 41 1193-1205.
http://rsif.royalsocietypublishing.org/content/6/41/1193.full
Adam Lacy-Hulberta, James C. Metcalfea, and Robin Hesketh, 1998: Biological responses to electromagnetic fields. The FASEB Journal. 1998;12:395-420. http://www.fasebj.org/cgi/content/full/12/6/395
E. Paluch, J. van der Gucht, and C. Sykes (2006): Cracking up: symmetry breaking in cellular systems. J. Cell Biol. 175, 687-692 http://jcb.rupress.org/content/175/5/687.abstract
Fatih M. Uckun, Tomohiro Kurosaki, Jizhong Jin, Xiao Jun, Andre Morgan, Minoru Takata, Joseph Bolen and Richard Luben, 1995: Exposure of B-lineage Lymphoid Cells to Low Energy Electromagnetic Fields Stimulates Lyn Kinase. November 17, 1995 The Journal of Biological Chemistry, 270, 27666-27670. doi: 10.1074/jbc.270.46.27666
Vijayanand Vajrala, James R. Claycomb, Hugo Sanabria, and John H. Miller, Jr., 2008: Effects of Oscillatory Electric Fields on Internal Membranes: An Analytical Model. Biophys J. 2008 March 15; 94(6): 2043–2052. doi: 10.1529/biophysj.107.114611. PMCID: PMC2257880 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2257880/?tool=pubmed
Vasily Vasilievitch Vorobyov, Evgeni Alekseevitch Sosunov, Nikolai Ilitch Kukushkin and Valeri Vasilievitch Lednev, 1998: Weak combined magnetic field affects basic and morphine-induced rat's EEG. Brain Research Volume 781, Issues 1-2, 19 January 1998, Pages 182-187. doi:10.1016/S0006-8993(97)01228-6
Carlo Venturaa, Margherita Maiolia, Gianfranco Pintusa, Giovanni Gottardic and Ferdinando Bersani, 2000: Elf-pulsed magnetic fields modulate opioid peptide gene expression in myocardial cells. Cardiovasc Res (2000) 45 (4): 1054-1064. doi: 10.1016/S0008-6363(99)00408-3
Hui Ye, Marija Cotic, Eunji E Kang, Michael G Fehlings, and Peter L Carlen, 2010: Transmembrane potential induced on the internal organelle by a time-varying magnetic field: a model study. J Neuroeng Rehabil. 2010; 7: 12. doi: 10.1186/1743-0003-7-12. PMCID:PMC2836366 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2836366/
Bagi bagi bonus sabung ayam online
SvaraRaderaRacikan Ramuan Tradisional Untuk Ayam Aduan
SvaraRaderaSenjata Mematikan Kaki Ayam Aduan Filipina